研究テーマ、得意領域
機能性ペプチドの構造化学的研究
ポリペプチドが生体内で多様な「働き」を担うためには、その目的に適した「かたち」をとる必要があります。これらのポリペプチドをナノメートルの世界で観察することによって、「かたち」と「働き」の関係を見つけ、生命活動の根源となる現象を原子のレベルで明らかにしようと取り組んでいます。
キーワード
配属学生
| 大学院生 | 0名 |
|---|---|
| 学部学生 | 6年次生:12名、5年次生:10名、4年次生:12名 |
所属教員
研究内容
例えば、L-Phe-L-Leuという配列は、抗菌作用をもつペプチド、鎮痛作用をもつペプチドなどのいろいろなペプチドに含まれています。このジペプチドは短いのでそれ自身に特別な作用はありませんが、典型的な芳香族アミノ酸(Phe)と典型的な枝分かれ構造をもつアミノ酸(Leu)の組合せなので、古くから基礎研究が行われてきたと考えられます。ところが、分子の構造が何十万件も登録されたデータベースを調べても、このジペプチドに関する情報は最近まで抜け落ちていました。
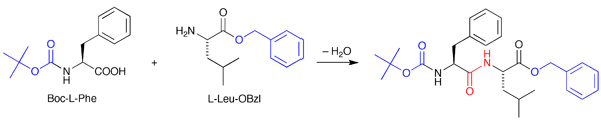
このジペプチドの構造を調べるためには、まず合成を行います。合成の利点は、生物が生成する量よりも多く、しかも高い純度で目的物を得られることです。L-Phe-L-Leuを合成する場合、L-Pheにt-butyloxycarbonyl基(以下、Bocと略す)を導入したBoc-L-Pheと、L-Leuにbenzyl ester基(以下、OBzlと略す)を導入したL-Leu-OBzlとを最初に合成します。このBoc基とOBzl基は保護基と呼ばれ、反応させたくない官能基を不活性化することで、余計な反応がおこるのを防ぎます。このBoc-L-PheとL-Leu-OBzlを適当な有機溶媒に溶かし、脱水反応をおこす試薬を加えると、アミド結合が形成され2残基のペプチドBoc-L-Phe-L-Leu-OBzlが合成できます(図1)。

このペプチドを再結晶し、0.3 mm角程度の単一な結晶を1つ取り出します(図2)。この結晶に波長の短い光(0.7〜1.5Å)をあてると、幾何学的な配列をした回折点が観測されます(図3)。幾何学的な配置と回折点の強度を一つずつ測定し、数学的な統計処理をすることで、結晶中の分子の立体構造を精密に知ることができます。(これを解析するといいます)
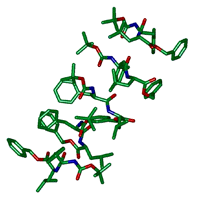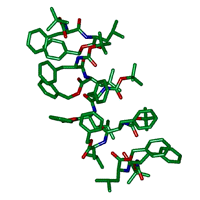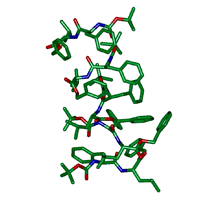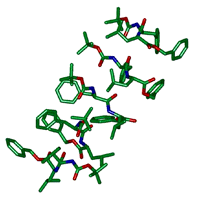
図4 結晶中に見つかった7つのペプチド分子
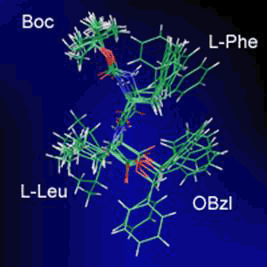
図5 7分子の重ね合わせ図
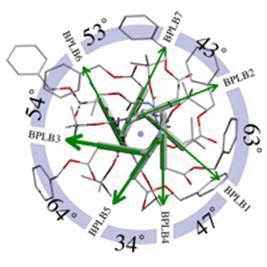
図6 図4を「上」から見た模式図
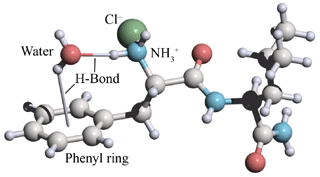
図7 水分子とL-Phe-L-Leu-NH2
図8 電子分布状態
このように、たった2つのアミノ酸が結合するだけでも、化学構造式からは想像もできないほど、立体構造はきれいで多様性に富んでいます。いくつもの類似化合物に対し、このような解析を行い、その結果解明されたそれら分子の「かたち」と機能と比較することで、機能性分子の設計に必要な基礎的情報を収集できます。
代表的論文
2024年
- Asano A, Nakayama K, Okada S, Kato T, Doi M: Puckering effects of 4-hydroxy-L-proline isomers on the conformation of ornithine-free Gramicidin S. Acta Crystallogr E Crystallogr Commun. 80:942, 2024.
- Asano A, Sakata M, Kato T, Doi M: Fluorine–hydrogen interactions observed in a helix structure having an orn-free gramicidin S sequence incorporating 4-trans -fluoroproline. Acta Crystallogr E Crystallogr Commun.81:345, 2025.
- Kato T, Ofuka G, Kobayashi R, Asano A, Doi M: Evaluation of peptide secondary structure and intracellular uptake by introducing disubstituted amino acids into the amphipathic helical peptide C18AA. Results in Chemistry 14:102103, 2025.
- Asano A, Kawanami Y, Fujita M, Yano Y, Ide R, Minoura K, Kato T, Doi M: Electronic substituent effect on the conformation of a phenylalanine-incorporated cyclic pepide.RSC Adv.,14:1062-1071, 2024.
2023年
- Ito T, Yokoo H, Kato T*, Doi M, Demizu Y: Sculpting Secondary Structure of a Cyclic Peptide: Conformational Analysis of a Cyclic Hexapeptide Containing a Combination of L-Leu, D-Leu, and Aib Residues. ACS Omega, 8:44106-44111, 2023.
- Uchiyama H, Ban K, Nozaki S, Ikeda Y, Ishimoto T, Fujioka H, Kamiya M, Amari R, Tsujino H, Arai M, Yamazoe S, Maekawa K, Kato T, Doi M, Kadota K, Tozuka Y, Tomita N, Sajiki H, Akai S, Sawama Y: Impact of multiple H/D replacements on the physicochemical properties of flurbiprofen.RSC Med Chem., 14:2583-2592, 2023.
2022年
- Kato T, Numa H, Nakamachi M, Asano A, Doi M: Effects of Substituting Disubstituted Amino Acids into the Amphipathic Cell Penetrating Peptide Pep-1. Chem. Pharm. Bull., 70:812–817, 2022.
- Ueda A, Makura Y, Kakazu S, Kato T, Umeno T, Hirayama K, Doi M, Oba M, Tanaka M: E-Selective Ring-Closing Metathesis in α-Helical Stapled Peptides Using Carbocyclic α,α-Disubstituted α-Amino Acids. Org. Lett., 24:1049–1054, 2022.
- Yokoo H, Misawa T, Kato T, Tanaka M, Demizu Y, Oba M: Development of delivery carriers for plasmid DNA by conjugation of a helical template to oligoarginine. Bioorg. Med. Chem., 72:116997, 2022.
2021年
- Asano A, Minami C, Matsuoka S, Kato T, Doi M: An Ornithine-Free Gramicidin S Analogue Using Norleucine, Cyclo(Val–Nle–Leu–D-Phe–Pro)2, Forms Helically Aligned β-Sheets. Chem. Pharm. Bull., 69:1097-1103, 2021.
- Makura Y, Ueda A, Kato T, Iyoshi A, Higuch, M, Doi M, Tanaka M. X-Ray Crystallographic Structure of α-Helical Peptide Stabilized by Hydrocarbon Stapling at i,i+1 Positions. Int. J. Mol. Sci., 22:5364, 2021.
- Sato K, Umeno T, Ueda A, Kato T, Doi M, Tanaka M. Asymmetric 1,4-Addition Reactions Catalyzed by N-Terminal Thiourea-Modified Helical l-Leu Peptide with Cyclic Amino Acids. Chemistry - A European Journal, 27:11216–11220, 2021.
- Yamaberi Y, Eto R, Umeno T, Kato T, Doi M, Yokoo H, Oba M, Tanaka M. Synthesis of (S)-(-)-Cucurbitine and Conformation of Its Homopeptides. Org. Lett., 23:4358–4362, 2021.
- Asano A, Nakagawa M, Miyajima C, Yasui M, Minoura K, Yamada T, Doi M. Effect of the Powerful Plasticity of the tert‐Butyl Side Chain on the Conformational Equilibrium of Ascidiacyclamides. J. Pept. Sci., e3363, 2021.
- Kato T, Kita Y, Iwanari K, Asano A, Oba M, Tanaka M, Doi M. Synthesis of Six-membered Carbocyclic Ring α,α-Disubstituted Amino Acids and Arginine-Rich Peptides to Investigate the Effect of Ring Size on the Properties of the Peptide. Bioorg. Med. Chem., 38:116111, 2021.
2020年
- Ueda A, Ikeda M, Kasae T, Doi M, Demizu Y, Oba M, Tanaka M: Synthesis of Chiral α‐Trifluoromethyl α,α‐Disubstituted α‐Amino Acids and Conformational Analysis of L‐Leu‐Based Peptides with (R)‐ or (S)‐α‐Trifluoromethylalanine. ChemistrySelect, 5: 10882-10886, 2020.
- Asano A, Minoura K, Kojima Y, Yoshii T, Ito R, Yamada T, Kato T, Doi M: NMR-based quantitative studies of the conformational equilibrium between their square and folded forms of ascidiacyclamide and its analogues. RSC Adv., 10: 33317-33326, 2020.
- Asano A, Yamada T, Doi M: Incorporation of β-amino acids into ascidiacyclamides: Effects on conformation, cytotoxicity and interaction with copper (II) ion. J. Pept. Sci., 12: e3225. 2020.
2019年
- Umeno T, Ueda A, Doi M, Kato T, Oba M, Tanaka M: Helical foldamer-catalyzed enantioselective 1, 4-addition reaction of dialkyl malonates to cyclic enones. Tertrahedron Let.,60: 151301, 2019.
- Asano A, Matsuoka S, Minami C, Kato T, Doi M.: [Leu2]Gramicidin S preserves the structural properties of its parent peptide and forms helically aligned β-sheets. Acta Cryst., C75: 1336-1343, 2019.
- Asano A, and Doi M: A bis-copper(II)–[D-βVal3,7]ascidiacyclamide complex enveloping two square pyramids and sharing an apex atom from a carbonate anion. Acta Cryst., C75: 1182-1187, 2019.
- Kato T, Kishimoto S, Asano A, Doi M: Crystal structure of N-{N-[N-(tert-butoxycarbonyl)- L-a-aspartyl]-L-a-aspartyl}-L-a-aspartic acid 14,24,34-trimethyl ester 31-2-oxo-2-phenylethyl ester {Boc-[Asp(OMe)]3-OPac}. Acta Cryst., E75: 585–588, 2019.
- Asano A and Doi M: Crystal Structure of Gramicidin S Hydrochloride at 1.1 Å Resolution. X-Ray Struct. Anal. Online, 35: 1-2, 2019.
2018年
- Tanaka M, Yakabi H, Nakatani H, Ueda A, Doi M, Oba M: Helical Structures of Cyclopentene- based α,α-Disubstituted α-Amino Acid Homopeptides. CHIMIA, 72: 848-852, 2018.
- Asano A, Yamada T, Taniguchi T, Sasaki M, Yoza K, Doi M: Ascidiacyclamides containing oxazoline and thiazole motifs assume square conformations and show high cytotoxicity. J Pept Sci., e3120, 2018.
- Koba Y, Ueda A, Oba M, Doi M, Kato T, Demizu Y, Tanaka M: Left-Handed Helix of Three-Membered Ring Amino Acid Homopeptide Interrupted by an N-H···Ethereal O-Type Hydrogen Bond. Org Lett., 20:7830-7834, 2018.
- Tsuji G, Misawa T, Doi M, Demizu Y: Extent of Helical Induction Caused by Introducing α-Aminoisobutyric Acid into an Oligovaline Sequence. ACS Omega, 3:6395–6399, 2018.
- Kato T, Oba M, Nishida K, Tanaka M: Cell-Penetrating Peptides Using Cyclic α,α-Disubstituted α-Amino Acids with Basic Functional Groups. ACS Biomater. Sci. Eng., 4: 1368-1376, 2018.
2017年
- Koba Y, Ueda A, Oba M, Doi M, Demizu Y, Kurihara M, Tanaka M: Helical L-Leu-Based Peptides Having Chiral Five-Membered Carbocyclic Ring Amino Acids with an Ethylene Acetal Moiety. ChemistrySelect, 2: 8108-8114, 2017
- Eto R, Oba M, Ueda A, Uku T, Doi M, Matsuo Y, Tanaka T, Demizu Y, Kurihara M, Tanaka M: Diastereomeric Right- and Left-Handed Helical Structures with Fourteen (R)-Chiral Centers. Chem. Eur. J., 23: 18120-18124, 2017
- Kato T, and Doi M: Crystal structure of 3-(4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacen-3-yl)propanoic acid. Acta Cryst., E73: 1974-1976, 2017
- Asano A, Numata S, Yamada T, Minoura K, Doi M: Conformational properties of ascydiacyclamide analogues with cyclic a-amino acids instead of oxazoline residues. Bioorg. Med. Chem., 25: 6554-6562, 2017
- Furukawa K, Oba, M, Toyama K, Opiyo G O, Demizu Y, Kurihara M, Doi M, Tanaka M: Low pH-triggering changes in peptide secondary structures. Org. Biomol. Chem., 15: 6302-6305, 2017

2016年
- Demizu Y, Doi M, Yamashita H, Misawa T, Oba M, Kurihara M, Suemune H, Tanaka M: The side-chain hydroxy groups of a cyclic α,α-disubstituted α-amino acid promote oligopeptide 310-helix packing in the crystalline state. Biopolymers, 5: 757-768, 2016
- Demizu Y, Okitsu K, Doi M, Misawa T, Oba M, Tanaka M, Kurihara M: Influence of L-Leu to D-Leu Replacement on the Helical Secondary Structures of L-Leu-Aib-Based Dodecapeptides. ChemistrySelect, 1: 5805-5811, 2016
- Demizu Y, Yamashita H, Misawa T, Doi M, Oba M, Tanaka M, Kurihara M: Handedness preferences of heterochiral helical peptides containing homochiral peptide segments. Eur. J. Org. Chem., 2016(4): 840-846, 2016
- Umeno T, Ueda A, Oba M, Doi M, Hirata T, Suemune H, Tanaka M: Helical structures of L-Leu-based peptides having chiral six-membered ring amino acids. Tetrahedron, 72(22): 3124-3131, 2016
- Ueda A, Oba M, Izumi Y, Sueyoshi Y, Doi M, Demizu Y, Kurihara M, Tanaka M: Helical structures of homo-chiral isotope-labeled α-aminoisobutyric acid peptides. Tetrahedron, 72(39): 5864-5871, 2016
- Yamaguchi T, Hoshino M, Miyachi K, Kamino S, Nakahara R, Doi M, Asano M, Matsumura H, Fujita Y: Crystal structure of o-carboxyphenylfluorone as a multifunctional dye. X-Ray Struct. Anal. Online, 32: 9-10, 2016
- Furukawa K, Oba M, Opiyo GO, Doi M, Tanaka M: Cyclic α,α-Disubstituted α-Amino Acids with Menthone in Their Side-Chains Linked through an Acetal Moiety and Helical Structures of Their Peptides. Eur. J. Org. Chem., : 2988–2998, 2016
- Demizu Y, Okitsu K, Yamashita H, Doi M, Misawa T, Oba M, Tanaka M, Kurihara M: α-Helical Structures of Oligopeptides with an Alternating L-Leu- Aib Segment. Eur. J. Org. Chem. , : 2815–2820, 2016
- Ueda A, Umeno T, Doi M, Akagawa K, Kudo K, Tanaka M.: Helical-Peptide-Catalyzed Enantioselective Michael Addition Reactions and Their Mechanistic Insights. J Org Chem, 81(15): 6343-6356, 2016
- Kawashima H, Katayama M, Yoshida R, Akaji K, Asano A, Doi M.: A dimer model of human calcitonin13-32 forms an α-helical structure and robustly aggregates in 50% aqueous 2,2,2-trifluoroethanol solution. J Pept Sci, 22(7): 480-484, 2016
- Demizu Y, Doi M, Yamashita H, Misawa T, Oba M, Kurihara M, Suemune H, Tanaka M.: The side-chain hydroxy groups of a cyclic α,α-disubstituted α-amino acid promote oligopeptide 310 -helix packing in the crystalline state. Biopolymers, 106(5): 757-768, 2016
- Asano A, Minoura K, Yamada T, Doi M.: Conformational transformation of ascidiacyclamide analogues induced by incorporating enantiomers of phenylalanine, 1-naphthylalanine or 2-naphthylalanine. J Pept Sci, 22(3): 156-165, 2016
- Koba Y, Hirata Y, Ueda A, Oba M, Doi M, Demizu Y, Kurihara M, Tanaka M.: Synthesis of chiral five-membered carbocyclic ring amino acids with an acetal moiety and helical conformations of its homo-chiral homopeptides. Bioplymers Peptide Sci, 106(4): 555-562, 2016
- Oba M, Nonaka H, Doi M, Tanaka M.: Conformational studies on peptides having dipropylglycine (Dpg) or 1-aminocycloheptanecarboxylic acid (Ac7c) within the sequence of L-leucine (Leu) residues. Biopolymers, 106: 210-218, 2016
2015年
- Demizu Y, Yamashita H, Doi M, Misawa T, Oba M, Tanaka M, Kurihara M: Topological Study of the Structures of Heterochiral Peptides Containing Equal Amounts of l-Leu and d-Leu. J. Org. Chem. 80:8597-8603, 2015
- Demizu Y, Misawa T, Yamagata N, Doi M, Kurihara T: Methyl 2-[(2-{2-[(2-acetamidophenyl)ethynyl]benzamido}phenyl)ethynyl]benzoate. Molbank 2015: 854, 2015
- Tanda K, Eto R, Kato K, Oba M, Ueda A, Suemune H, Doi M, Demizu Y, Kurihara M, Tanaka M: Peptide foldamers composed of six-membered ring α,α-disubstituted α-amino acids with two changeable chiral acetal moieties. Tetrahedron 71:3909-3914, 2015
- Hirata T, Ueda A, Oba M, Doi M, Demizu Y, Kurihara M, Nagano M, Suemune H, Tanaka M: Amino equatorial effect of a six-membered ring amino acid on its peptide 310- and α-helices. Tetrahedron 71:2409-2420. 2015
- Demizu Y, Yamashita H, Misawa T, Doi M, Tanaka M, Kurihara M: Effects of D-Leu residues on the helical secondary structures of L-Leu-based nonapeptides. Chem. Pharm. Bull. 63:218-224, 2015
2014年
- Asano M, Doi M, Baba K, Taniguchi M, Shibano M, Tanaka S, Sakaguchi M, Takaoka M, Hirata M, Yanagihara R, Nakahara R, Hayashi Y, Yamaguchi T, Matsumura H, Fujita Y: Bio-imaging of hydroxyl radicals in plant cells using the fluorescent molecular probe rhodamine B hydrazide, without any pretreatment. J. Biosci. Bioeng. 118:98-100, 2014
- Oba M, Kawabe N, Takazaki H, Demizu Y, Doi M, Kurihara M, Suemune H, Tanaka M: Conformational studies on peptides having chiral five-membered ring amino acid with two azido or triazole functional groups within the sequence of Aib residues. Tetrahedron 70:8900-8907, 2014
- Asano A, Yamada T, Doi M: Modulating the structure of phenylalanine-incorporated ascidiacyclamide through fluorination. J. Pept. Sci. 20:794-802, 2014
- Oba M, Takazaki H, Kawabe N, Doi M, Demizu Y, Kurihara M, Kawakubo H, Nagano M, Suemune H, Tanaka M: Helical Peptide-foldamers having a chiral five-membered ring amino Acid with two azido functional groups. J. Org. Chem. 79:9125-9140, 2014
- Hoshino M, Kamino S, Doi M, Takada S, Mitani S, Yanagihara R, Asano M, Yamaguchi T, Fujita Y: Spectrophotometric determination of hydrogen peroxide with osmium(VIII) and m-carboxyphenylfluorone. Spectrochim Acta, Part A, 117:814-816, 2014
2013年
- Demizu Y, Yamashita H, Yamazaki N, Sato Y, Doi M, Tanaka M, Kurihara M: Oligopeptides with Equal Amounts of L- and D-Amino Acids May Prefer a Helix Screw Sense. J. Org. Chem. 78:12106-12113, 2013
- Yamazaki N, Demizu Y, Sato Y, Doi M, Kurihara M: Helical Foldamer Containing a Combination of Cyclopentane-1,2-diamine and 2,2-Dimethylmalonic Acid. J. Org. Chem. 78:9991-9994, 2013
- Demizu Y, Nagoya S, Shirakawa M, Kawamura M, Yamagata N, Sato Y, Doi M, Kurihara M: Development of stapled short helical peptides capable of inhibiting vitamin D receptor (VDR)-coactivator interactions. Bioorg. Med. Chem. Lett. 23:4292-4296, 2013
- Nakanishi K, Doi M, Usami Y, Amagata T, Minoura K, Tanaka R, Numata A, Yamada T: Anthcolorins A-F, novel cytotoxic metabolites from a sea urchin-derived Aspergillus versicolor. Tetrahedron 69:4617-4623, 2013
- Oba M, Shimabukur, A, Ono M, Doi M, Tanaka M: Synthesis of both enantiomers of cyclic methionine analogue: (R)- and (S)-3-aminotetrahydrothiophene-3-carboxylic acids. Tetrahedron Asymmetry, 24:464-467, 2013