研究テーマ
分子間相互作用に基づく機能性粒子調製
キーワード
配属学生
| 大学院生 | 8名(薬学専攻D4:1名、D2:2名、薬科学専攻D3:3名、D1:2名) |
|---|---|
| 学部学生 | 6年次生:12名、5年次生:13名、4年次生:12名 |
所属教員
-
戸塚 裕一
教授 / 博士(薬学)
■担当科目
早期体験学習(1年)、物理薬剤学(2、3年)、薬学連携演習14(2年)、製剤設計学(3年)、薬剤学実習(3年)、特別演習・実習(4年~6年)、
特別演習(PBL)(大学院)、特別研究(大学院)
■所属学会および社会活動
日本薬学会、日本薬剤学会(代議員)、製剤機械技術学会(評議員)、粉体工学会、シクロデキストリン学会、日本海水学会、日本食品科学工学会、日本食品化学学会、創剤フォーラム、文部科学省 科学技術予測センター NISTEP専門調査員 -
内山 博雅
講師 / 博士(薬学)
■担当科目
物理薬剤学(2、3年)、薬学連携演習14(2年)、製剤設計学(3年)、薬剤学実習(3年)、医工学連携授業、(2年)、特別演習・実習(4年~6年)、
特別研究(大学院)
■所属学会および社会活動
日本薬剤学会、日本薬学会、製剤機械技術学会、粉体工学会、日本食品科学工学会、日本食品化学学会 -
田仲 涼眞
助教 / 博士(薬科学)
■担当科目
薬剤学実習(3年)、薬学連携演習14(2年)、特別演習・実習(4年~6年)、
特別研究(大学院)
■所属学会および社会活動
日本薬学会、日本薬剤学会、製剤機械技術学会、粉体工学会
研究内容
(1)ナノコンポジット形成に基づく難水溶性化合物の溶解性改善及び吸収性改善
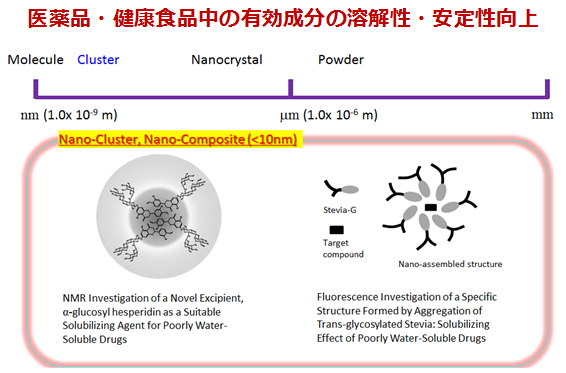
医薬品開発や特定機能性食品開発において、対象とする有効成分の水への溶解度が極めて低い場合には、優れた効果・効能を持っていても有効活用が困難となります。難水溶性有効成分を機能性添加剤と分子レベルで特異的に相互作用させ、その性質を保持した機能性粉末を調製することにチャレンジしています。溶解性や吸収性および安定性に優れた粉体の設計、次世代の特定保健食品開発のための基礎研究、医薬品を含む有機ナノ粒子の調製に取り組んでいます。
溶解度上昇の方法としては、薬物をアモルファス化あるいは高分子や糖類中に固体分散体化させる方法が知られています。そこで、新たな固体分散体調製法の確立や薬物のアモルファス分散状態についての物性評価法の確立や長期安定性の予測などについても取り組んでいます。
(2)機能性素材を用いた吸入用粒子の創成
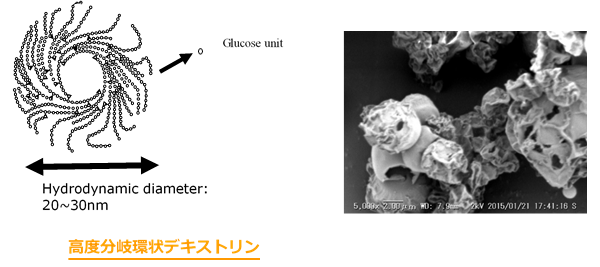
また、近年経口投与や注射による投与に代わる新たな投与経路として注目を集めている経肺投与製剤の開発にも取り組んでいます。中でも我々の研究室では、食品添加剤として利用されている高度分岐環状デキストリンが水中で20~30nmの均一なナノ構造に着目して、ナノスフェアから構成される経肺治療用の新しい製剤設計を試みています。多剤耐性緑膿菌などによる難治性肺炎治療などが最終的なターゲットであり、肺到達性が高く、組織障害性の低い吸入用粉末合剤のための基礎研究を行っています。
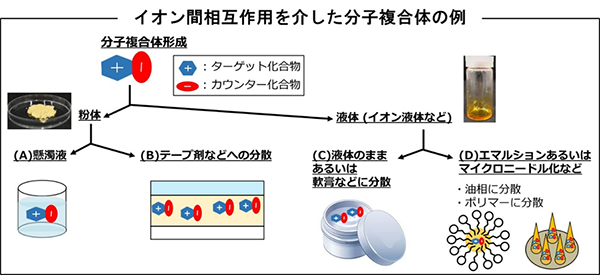
(3)医薬品が形成する分子複合体の創成とその評価
(4)機能性食品成分の可溶化および安定化を可能にする乳化法の開発
近年、医療費の高騰によりセルフメディケーションが推進される中、機能性食品に注目が集まっています。機能性食品成分の中には、体内での溶解性が低いものや、水中での保存安定性が低いものが多く存在します。我々の研究室では、溶けないあるいは保存安定性の低い化合物に対して、自己乳化製剤や油性製剤などの乳化技術を検討しています。自己乳化製剤では、食品添加剤のみを用いて数十ナノメートルの大きさのエマルション調製に成功し、機能性食品成分の吸収性改善や保存安定性の向上といった知見が得られています。
(5)その他
その他の研究として、新規固体分散体調製法の確立及び医薬品のアモルファス分散状態の探索や粉体プロセスによる機能性材料の創製や新たな粉体プロセスの構築など、他大学との共同研究、民間企業との産学共同研究を実施しています。
代表的論文
2024年
- T. Kämäräinen, Y. Nakayama, H. Uchiyama, Y. Tozuka, K. Kadota, Amyloid Nanofibril-Assisted Spray Drying of Crumpled Supraparticles, Small, 2309645 (2024)
- T. Komai, T. Togo, H. Uchiyama, K. Kadota, Y. Tozuka, Kinetics of crystal transformation of imatinib mesylate during formulation process: Selection of dry granulation in pharmaceutical process, Powder Technol., 438, 119570 (2024)
- S. Fujita, K. Kadota, A. Koike, H. Uchiyama, Y. Tozuka, S. Tanaka, “Wash-free” synthesis of cyclodextrin metal-organic frameworks, RSC Mechanochemistry, 1,153-157 (2024)
- T. Kämäräinen, S. Nogami, H. Arima-Osonoi, H. Iwase, H. Uchiyama, Y. Tozuka, K. Kadota, Multiscale structure analysis of a pH-responsive gelatin/hydroxypropyl methylcellulose phthalate blend using small-angle scattering. J Colloid Interface Sci., 669, 975-983 (2024)
- H. Uchiyama, K. Minoura, E. Yamada, K. Ando, R. Yamauchi, A. Nakanishi, M. Tandia, K. Kadota, Y. Tozuka, Solubilization mechanism of α-glycosylated naringin based on self-assembled nanostructures and its application to skin formulation, Eur. J. Pharm. Biopharm., 200, 114316 (2024)
- K. Semba, K. Kadota, T. Kämäräinen, Y. Nakayama, Y. Hatanaka, H. Uchiyama, H. Arima-Osonoi, K. Sugiyama, Y. Tozuka, Tailored Sugar-Mediated Porous Particle Structures for Improved Dispersion of Drug Nanoparticles in Spray-Freeze-Drying, Langmuir, 40, 14440-14454 (2024)
- P. Mo, Y. Hatanaka, T. Kämäräinen, S. Furukawa, M. Takase, S. Yamanaka, M. Doi, H. Uchiyama, K. Kadota*, Y. Tozuka, Cocrystal Formulation Design of 4-Aminosalicylic Acid and Isoniazid via Spray-drying Based on a Ternary Phase Diagram toward Simultaneous Pulmonary Delivery, Powder Technol., 445, 120126 (2024)
- K. Kadota, H. Uchiyama, T. Kämäräinen, S. Tanaka, Y. Tozuka, Building Respirable Powder Architectures: Precision in Particle Morphology Engineering for Enhanced Pulmonary Drug Delivery, Expert Opin. Drug Deliv., 21, 945–963(2024)
- K. Ando, H. Uchiyama, K. Minoura, K. Kadota, Y. Tozuka, The impact of adding a cationic metal salt and curcumin to monoammonium glycyrrhizic acid on its solubilizing capacity and gelation, Chem. Pharm. Bull., 72, 838-844 (2024)
- K. Sugimoto, H. Uchiyama, K. Kadota, K. Yuki,Y. Tozuka, Elucidating the Factors Affecting the Patient-centric Usability of Blister Packs for Spherical Capsules, Chem. Pharm. Bull., 72, 1048-1054 (2024)
2023年
- T. Kämäräinen, K. Kadota, J. Y. Tse, H. Uchiyama, T. Oguchi, H. Arima-Osonoi, Y. Tozuka, Tuning phytoglycogen size and aggregate structure with solvent quality: Influence of water-ethanol mixtures revealed by X-ray and light scattering techniques, Biomacromolecules, 24, 225−237 (2023)
- K. Ban, K. Imai, S. Oyama, J. Tokunaga, Y. Ikeda, H. Uchiyama, K. Kadota, Y. Tozuka, S. Akai, Y. Sawama, Alkyl Sulfonium Salt-based Reagents for Introduction of Deuterated Alkyl Groups in Drug Discovery, Angew. Chem. Int. Ed., 62(48), e202311058 (2023)
- H. Uchiyama, K. Ban, S. Nozaki, Y. Ikeda, T. Ishimoto, H. Fujioka, M. Kamiya, R. Amari, H. Tsujino, M. Arai, S. Yamazoe, K. Maekawa, T. Kato, M. Doi, K. Kadota, Y. Tozuka, N. Tomita, H. Sajiki, S. Akai, Y. Sawama, Impact of Multiple H/D Replacement on the Physicochemical Properties of Flurbiprofen, RSC Medicinal Chemistry, 14, 2583 (2023)
- H. Uchiyama, K. Kadota, Y. Tozuka, A review of transglycosylated compounds as food additives to enhance the solubility and bioavailability of active components with poor aqueous solubility, Crit. Rev. Food Sci. Nutr., 63(32), 11226-11243 (2023)
- Y. Hatanaka, H. Uchiyama, S. Kaneko, K. Ueda, K. Higashi, K. Moribe, S. Furukawa, M.Takase, S. Yamanaka, K. Kadota, Y. Tozuka, Designing a novel coamorphous salt formulation of telmisartan with amlodipine to enhance permeability and oral absorption, Mol. Pharm., 20, 4071-4085 (2023)<IF:5.364>
- K.Kadota, J. Y. Tse, Jun Yee, S. Fujita, N. Suzuki, H. Uchiyama, Y. Tozuka, S. Tanaka, Drug-facilitated crystallization of spray-dried CD-MOFs with tunable morphology, porosity, and dissolution profile, ACS Appl. Bio Mater., 6, 3451-3462 (2023)
- T. Kämäräinen, K. Kadota, H. Arima-Osonoi, H. Uchiyama, Y. Tozuka, Tailoring the Self-Assembly of Steviol Glycoside Nanocarriers with Steroidal Amphiphile, ACS Biomater. Sci. Eng., 9, 5747-5760 (2023)
- H. Uchiyama, Y. Hanamoto, Y. Hatanaka, K. Kadota, Y. Tozuka, The enhanced skin permeation of flavonoids via the application of a coamorphous in a microemulsion formulation, J.Pharm. Sci., 112, 3067-3074 (2023)
- K. Kadota, T. Kämäräinen, F. Sakuma, K. Ueda, K. Higashi, K. Moribe, H. Uchiyama, K. Minoura, Y. Tozuka, Unveiling the solubility enhancement of flavone via α-glucosyl rutin and hesperidin: Probing the structural differences through NMR and SAXS analysis, Food Funct., 14, 10493-10505 (2023)
- H. Ueda, Y. Hirakawa, T. Miyano, Y. Nakayama, Y. Hatanaka, H. Uchiyama, Y. Tozuka, K. Kadota, Improvement in Inhalation Properties of Theophylline and Levofloxacin by Co-Amorphization and Enhancement in Its Stability by Addition of Amino Acid as a Third Component, Mol. Pharm., 20, 6368-6379 (2023)
- Y. Hatanaka, H. Uchiyama, S. Furukawa, M. Takase, S. Yamanaka, K. Kadota, Y. Tozuka, Effect of solubility improvement via formation of an amorphous composite of indomethacin and sulindac on membrane permeability, Chem. Pharm. Bull., 71, 257–261 (2023)
- M. Fukada, K. Kadota, S. Nogami, H. Uchiyama, Y. Shirakawa, Y. Tozuka, Development of bitter-taste masked instant jelly formulations of diphenhydramine hydrochloride with easy-to-consume granules, Chem. Pharm. Bull., 71, 670–674 (2023)
2022年
- X. Ma, K. Higashi, K. Fukuzawa, K. Ueda, K. Kadota, Y. Tozuka, E. Yonemochi, K. Moribe, Computational approach to elucidate the formation and stabilization mechanism of amorphous formulation using molecular dynamics simulation and fragment molecular orbital calculation, Int. J. Pharm., 615, 121477 (2022)
- J. Y. Tse, K. Kadota, T. Nakajima, H. Uchiyama, S. Tanaka, Y. Tozuka, Crystalline rearranged CD-MOF particles obtained via spray-drying synthesis applying to inhalable formulations with high drug loading, Cryst. Growth. Des., 22, 1143-1154 (2022)
- K. Kadota, K. Matsumoto, H. Uchiyama, S. Tobita, M. Maeda, D. Maki, Y. Kinehara, I. Tachibana, T. R. Sosnowski, Y. Tozuka, In silico evaluation of particle transport and deposition in the airways of individual patients with chronic obstructive pulmonary disease, Euro. J. Pharm. Biopharm., 174, 10-19 (2022)
- H. Ueda, Y. Hirakawa, T. Miyano, M. Imono, J. Y. Tse, H. Uchiyama, Y. Tozuka, K. Kadota, Design of a stable coamorphous system using lactose as an anti-plasticizing agent for diphenhydramine hydrochloride with a low glass transition temperature, Mol. Pharm., 19, 1209-1218 (2022)
- T. Kämäräinen, K. Kadota, J. Y. Tse, H. Uchiyama, S. Yamanaka, Y. Tozuka, Modulating Pore Space Architecture of Ice-Templated Dextran Microparticles Using Molecular Weight and Concentration, Langmuir, 38, 6741-6751 (2022)
- Y. Murase, K. Takayama, T. Uchimoto, H. Uchiyama, K. Kadota, Y. Tozuka, Prediction of tablet weight variability from bulk flow properties by sparse modeling, Powder Technol., 407, 117681
- Y. Hatanaka, H. Uchiyama, K. Kadota, Y. Tozuka, Designing amorphous formulations of polyphenols with naringin by spray-drying for enhanced solubility and permeability, Adv. Powder Technol., 33, 103627 (2022)
- H. Uchiyama, S. Asai, A. Nakanishi, M. Tandia, K. Kadota, Y. Tozuka, The usability of transglycosylated stevia for the preparation of an oil-in-water submicron emulsion using a high-pressure homogenizer, Food Sci. Technol. Res., 28 (5), 343–350, 2022
- S. Nogami, K. Kadota, H. Uchiyama, H. Arima-Osonoi, M. Shibayama, Y. Tozuka, Rheological and rupture properties of gelatin-based hydrogels formulated with polymers for drug diffusion behavior, Polymer journal, 54, 1477-1487 (2022)
2021年
- S. Nogami, H. Uchiyama, K. Kadota, Y. Tozuka, Design of pH-responsive oral gel formulation based on the matrix systems of gelatin/hydroxypropyl methylcellulose phthalate toward controlled drug release, Int. J. Pharm., 592, 120047 (2021)
- J. Y. Tse, K. Kadota, T. Imakubo, H. Uchiyama, Y. Tozuka, Enhancement of the extra-fine particle fraction of levofloxacin embedded in excipient matrix formulations for dry powder inhaler using response surface methodology, Euro. J. Pharm. Sci., 156, 105600 (2021)
- H. Uchiyama, T. Ando, K. Kadota, Y. Tozuka, Enhanced solubility in both oil components and aqueous media via the formation of an amorphous composite between naringenin and hesperetin, J. Drug Deliv. Sci. Tech., 62, 102410 (2021)
- C. Aoki, X. Ma, K. Higashi, Y. Ishizuka, K. Ueda, K. Kadota, K. Fukuzawa, Y. Tozuka, K. Kawakami, E. Yonemochi, K. Moribe,Stabilization mechanism of amorphous carbamazepine by transglycosylated rutin, a non-polymeric amorphous additive with high glass transition temperature, Int. J. Pharm., 600, 120491(2021)
- S. Nogami, K. Minoura, N. Kiminami, Y. Kitaura, H. Uchiyama, K. Kadota, Y. Tozuka, Effect of the addition of cyclodextrin to spray-dried particles of curcumin/polyvinylpyrrolidone on the supersaturated state of curcumin, Adv. Powder. Technol., 32, 1750-1756 (2021)
- K. Kadota, H. Terada, A. Fujimoto, S. Nogami, H. Uchiyama, Y. Tozuka, Formulation and evaluation of bitter taste-masked orally disintegrating tablets of high memantine hydrochloride loaded granules coated with polymer via layering technique, Int. J. Pharm., 604, 120725 (2021)
- R. Okura*, H. Uchiyama, K. Kadota, Y. Tozuka, Hydrogen bonding from crystalline water mediates the hydration/dehydration of mequitazine glycolate, Cryst. Eng. Comm., 23, 4816 (2021)
- Y. Hatanaka, H. Uchiyama, K. Kadota, Y. Tozuka, Improved solubility and permeability based on nifedipine-ketoconazole coamorphous formation with simultaneous dissolution behavior, J. Drug Deliv. Sci. Tech., 65, 102715 (2021).
- J. Y. Tse, A. Koike, K. Kadota, H. Uchiyama, K. Fujimori, Y. Tozuka, Porous Particles and Novel carrier particles with enhanced penetration for efficient pulmonary delivery of antitubercular drugs, Euro.J. Pharm. Biopharm.,167, 116-126 (2021)
- S. Nogami, K. Kadota, H. Uchiyama, H. Arima, H. Iwase, T. Tominaga, T. Yamada, S. Takata, M. Shibayama, Y. Tozuka, Structural changes in pH-responsive gelatin/hydroxypropyl methylcellulose phthalate blends aimed at drug-release systems, Int. J. Biol. Macromol., 190, 989-998 (2021)
2020年
- K. Kadota, S. Nogami, H. Uchiyama, Y. Tozuka, Controlled dissolution behavior of curcumin in a kappa-carrageenan gel with metal chloride-mediated flexible texture, Food Hydrocolloids, 101, 105564 (2020)
- H. Uchiyama, M. Dowaki, K. Kadota, H. Arima, K. Sugiyama, Y. Tozuka, Single-stranded β-1,3–1,6-glucan as a carrier for improved the dissolution and membrane permeation of poorly water-soluble compounds, Carbohydr. Polym., 116698, 247 (2020)
- K. Kadota, T.R. Sosnowski, S.Tobita, I. Tachibana, J.Y. Tse, H. Uchiyama, Y. Tozuka, A particle technology approach toward designing dry-powder inhaler formulations for personalized medicine in respiratory diseases, Adv. Powder. Technol., 31, 219-226 (2020)
- A. Srivastava, H. Uchiyama, H. Imano, H. Satone, K. Iimura, K. Kadota, Y. Tozuka, Enhanced micellization of Gemini surfactants using diphenhydramine hydrochloride as an organic counterion, J. Mol. Liq., 300, 112288 (2020)
- K. Kadota, N. Inoue, Y. Matsunaga, T. Takemiya, K. Kubo, H. Uchiyama, Y. Tozuka, Numerical simulations of particle behavior in a respiratory system with varying inhalation patterns, J. Pharm. Pharmacol., 72, 17-28 (2020)
- M. Imono, H. Uchiyama, S. Yoshida, S. Miyazaki, N. Tamura, H. Tsutsumimoto, K. Kadota, Y. Tozuka, The elucidation of the key factor for oral absorption enhancement of nanocrystal formulations: In vitro - in vivo correlation of nanocrystals, Euro. J. Pharm. Biopharm., 146, 84-92 (2020)
- K. Kadota, T. Ibe, Y. Sugawara, Y. A. Yusof, H. Uchiyama, Y. Tozuka, S. Yamanaka, Production of mesoporous calcium carbonate with controlled specific surface area and its potential to ferulic acid release, RSC advances., 10, 28019 (2020)
- R. Okura, H. Uchiyama, K. Kadota, Y. Tozuka, New Salt and Cocrystal of Mequitazine: Impact of Coformer Flexibility and Hydrogen Bonding Donor on Polymorphism, Cryst. Growth. Des., 20, 7219-7229 (2020)
- K. Kadota, M. Tanaka, H. Nishiyama, J. Y. Tse, H. Uchiyama, Y. Shirakawa, Y. Tozuka, An effective approach to modifying inhalable betamethasone powders by morphology and surface control with a bio-surfactant, Powder Technol., 376, 517-526 (2020)
- A. Kitayama, K. Kadota, Y. Tozuka, A. Shimosaka, M. Yoshida, Y. Shirakawa, Molecular aspects of glycine clustering and phase separation in an aqueous solution during anti-solvent crystallization, Cryst. Eng. Comm., 22, 5182 (2020)
2019年
- K. Kadota, Y. Yanagawa, T. Tachikawa, Y. Deki, H. Uchiyama, Y. Shirakawa, Y. Tozuka, Development of porous particles using dextran as an excipient for enhanced deep lung delivery of rifampicin, Int. J. Pharm., 555, 280-290 (2019)
- A. Srivastava, H. Uchiyama, Y. Wada, Y. Hatanaka, Y. Shirakawa, K. Kadota, Y. Tozuka, Mixed micelles of the drug diphenhydramine hydrochloride with anionic and non-ionic surfactants show improve solubility, drug release and cytotoxicity of ethenzamide, J. Mol. Liq., 277, 349-359 (2019)
- H. Uchiyama, Y. Wada, Y. Hatanaka, Y. Hirata, M. Taniguchi, A. Nakanishi, M. Tandia, K. Kadota, Y. Tozuka, Enhanced solubility and permeability of quercetin by composite formation between α-glucosyl stevia and hydrophilic polymer, J. Pharm. Sci., 108, 2033-2040 (2019)
- M. Imono, H. Uchiyama, N. Sato, K. Kadota, Y. Tozuka, Real time dissolution and permeation studies of nanocrystal formulations by second derivative UV spectroscopy: The mechanism of its permeability enhancement effect by nanocrystallization, Int. J. Pharm., 558, 242-249 (2019)
- H. Uchiyama, K. Kadota, A. Nakanishi, M. Tandia, Y. Tozuka, A simple blending with α-glycosylated naringin produce the enhancement of solubility and absorption of pranlukast hemihydrate, Int. J. Pharm., 567, 118490 (2019)
- K. Semba, K. Kadota, H. Arima, A. Nakanishi, M. Tandia, H. Uchiyama, K. Sugiyama, Y. Tozuka, Improved water dispersibility and photostability in folic acid nanoparticles with transglycosylated naringin using combined processes of wet-milling and freeze-drying, Food Res. Int., 121, 108-116 (2019)
- J. Y. Tse, K. Kadota, Z. Yang, H. Uchiyama, Y. Tozuka, Investigation of the molecular state of 4-aminosalicylic acid in matrix formulations for dry powder inhalers using solid-state fluorescence spectroscopy of 4-dimethylaminobenzonitrile, Adv. Powder. Technol., 30, 2422-2429 (2019)
- S. Tanida, A. Yoshimoto, M. Yoshida, H. Uchiyama, K. Kadota, Y. Tozuka, Preparation of amorphous composite particles of drugs with ursodeoxycholic acid as preclinical formulations, Chem. Pharm Bull, 67, 921-928 (2019)
- H. Uchiyama, J. Chae, K. Kadota, Y. Tozuka, Formation of food grade microemulsion with rice glycosphingolipids to enhance the oral absorption of Coenzyme Q10, Foods., 8, 502 (2019)
- C. Mori, K. Kadota, Y. Tozuka, A. Shimosaka, M. Yoshida, Y. Shirakawa*, A novel powderization process of microcapsulated soybean oil using nozzleless electrostatic atomization, J. Food Eng., 246, 25-32 (2019)
2018年
- H. Uchiyama, A. Srivastava, M. Fujimori, K. Tomoo, A. Nakanishi, M. Tandia, K. Kadota, Y. Tozuka, Investigation of physiological properties of transglycosylated stevia with cationic surfactant and its application to enhance the solubility of rebamipide, J. Phys. Chem. B., 122, 10051-10061 (2018)
- K. Kadota, H. Arima, R. Shakudo, K. Semba, H. Uchiyama, K. Sugiyama, Y. Tozuka, Emergent composite structures following the amorphization of itraconazole with transglycosylated rutin by over-grinding, Powder Technol., 323, 69-75 (2018)
- H. Uchiyama, S. Nogami, K. Katayama, K. Hayashi, K. Kadota, Y. Tozuka, Jelly containing composite based on α-glucosyl stevia and polyvinylpyrrolidone: Improved dissolution property of curcumin, Euro. J. Pharm.Sci, 117, 48-54 (2018)
- K. Kadota, M. Hashimoto, T. Yamaguchi, H. Kawachi, H. Uchiyama, Y. Tozuka, Preparation of a highly water-dispersible powder containing hydrophobic polyphenols derived from chrysanthemum flower with a xanthine oxidase-inhibitory activity, Food Sci. Technol. Res., 24, 273-281 (2018)
- H. Uchiyama, Y. Wada, M. Takamatsu, K. Kadota, Y. Tozuka, Improved dissolution property of quercetin by preparing evaporated particles with transglycosylated rutin and isoquercitrin, Environ. Control Biol., 56, 161-165 (2018)
- K. Kadota, A. Imanaka, M. Shimazaki, T. Takemiya, K. Kubo, H. Uchiyama, Y. Tozuka, Effects of inhalation procedure on particle behavior and deposition in the airways analyzed by numerical simulation, J. Taiwan. Inst. Chem. Eng., 90, 44-50 (2018)
- J. Y. Tse, K. Kadota, T. Takeo, Y. Hirata, M. Taniguchi, H. Uchiyama, Y. Tozuka, Characterization of matrix embedded formulations for combination spray-dried particles comprising pyrazinamide and rifampicin, J. Drug Deliv. Sci. Tech., 48, 137-144 (2018)
- A. Kitayama, K. Kadota, Y. Konishi, H. Uchiyama, Y. Tozuka, A. Shimosaka, M. Yoshida, Y. Shirakawa*, Amorphization progression of indomethacin during co-grinding with cystine from energy assessed by discrete element method simulation, J. Ind. Eng. Chem., 62, 436-445 (2018)
- Y. Deki, K. Kadota, S. Onda, Y. Tozuka, A. Shimosaka, M. Yoshida, Y. Shirakawa, Crystallization behavior of glycine molecules with electrolytic dissociation on charged silica gel particles, Chem. Eng. Technol., 41, 1073-1079 (2018)
- S. Tanida, N. Takata, R. Takano, A. Sakon, T. Ueto, K. Shiraki, K. Kadota, Y. Tozuka, M. Ishigaki, Cocrystal structure design based on isomorphism, Cryst. Eng. Comm., 20, 362-369 (2018)
- N. Takata, S. Tanida, S. Nakae, K. Shiraki, Y. Tozuka, M. Ishigai, Tofogliflozin salt cocrystals with sodium acetate and potassium acetate, Chem. Pharm. Bull., 66, 1035-1040 (2018)