研究テーマ
精神・神経疾患における病態の分子基盤解明と、その診断・治療・個別化医療への応用 — 分子から「こころ」を読み解く、基礎と臨床の橋渡し研究 —
■ 研究室紹介
私たちの研究室では、アルツハイマー病を中心に、その病態分子機構の解明と診断・治療への応用を目指した研究を行っています。この研究を進める中で、神経系に関わる様々な分子の基礎的な解析にも取り組むようになり、その成果を踏まえて研究対象はさらに広がっています。現在では、アルツハイマー病以外の認知症、自己免疫性脳炎、自閉スペクトラム症(ASD)、統合失調症、うつ病などの精神・神経疾患、さらには一部の腫瘍や生活習慣病にも研究領域を拡大しています。分子生物学・生化学的手法を用いた実験研究を中心に、疾患の背景にあるメカニズムの解明や、創薬シーズの探索に取り組んでいます。
また、副作用報告データベースを活用したリアルワールドデータ解析を通じて、精神・神経疾患領域における薬物治療の安全性評価や、同種薬間における最適な治療選択肢の検討も行っています。こうしたデータ駆動型の研究は、臨床現場でのリスクマネジメントや個別化医療の実現に資するエビデンスの創出につながると考えています。
精神疾患はしばしば「見えにくい病」と言われますが、当研究室では、分子レベルの変化と認知機能・行動との関連を橋渡しすることで、「こころ」の変調を科学的に捉えることを目指しています。このような研究では、臨床心理士・公認心理師としての知見も活かしながら、心と脳のあいだにある複雑なメカニズムの理解と、それに基づく臨床・教育への還元を重視しています。
さらに、臨床現場で生まれる問いに応える形で、薬学的・化学的アプローチによる病態関連分子の検出・可視化技術の開発にも力を注いでいます。特定の疾患にとどまらず、複数疾患に共通する分子ネットワークや異常の検出を視野に入れ、バイオマーカーや診断補助ツールの開発を通じて、基礎と臨床をつなぐトランスレーショナルリサーチを推進しています。
本研究室では、こうした研究活動を通じて、病態を科学的に捉える力と、それを実際の医療につなげて考える思考力の養成を目指しています。将来、病院薬剤師をはじめとする薬学分野の幅広いフィールドで活躍できる人材の育成に貢献したいと考えています。
なお、本研究室に所属する学部学生の研究実績として、日本薬学会において、2024年には3名(https://www.ompu.ac.jp/news/20240521_p.html)、2025年には4名(https:// www.ompu.ac.jp/news/20250515_p.html)の学部学生が学生優秀発表賞を受賞しています。
キーワード
精神神経疾患、アルツハイマー病を含む認知症、自己免疫性脳炎、自閉スペクトラム症、うつ病、統合失調症、認知行動療法、薬物有害事象解析、分子病態バイオマーカー、アミロイドβ、γセクレターゼ、分子精神医学、化学的検出技術、光親和性クロスリンク、非天然アミノ酸の遺伝的取り込み、フルオレセイン誘導体、キサンチン誘導体、エクソサイト、心理・行動症状の生物学的基盤
配属学生
| 大学院生 | 2名 |
|---|---|
| 学部学生 | 6年次生:13名、5年次生:13名、4年次生:16名 |
所属教員
-
福森 亮雄
教授 / 医学博士
■担当科目
早期体験学習2(1年)、薬物治療学3(3年)、アドバンスト薬物治療学1(4年)、病態・薬物治療学演習(4年)、特別演習・実習(4年~6年)、アドバンスト薬物治療学2(6年)、薬学総合演習(6年)
外国文献購読(大学院生)、病態解析学特論I(大学院)、病態評価演習(大学院生)
■所属学会および社会活動
日本薬学会、日本精神神経学会、日本認知症学会、日本認知療法・認知行動療法学会、日本認知・行動療法学会、日本老年医学会、日本分子生物学会、日本産業精神保健学会、精神保健指定医・精神科専門医・臨床心理士・公認心理師 -
山口 敬子
講師 / 博士(薬学)
■担当科目
早期体験学習1(1年)、早期体験学習2(1年)、薬物治療学2(2年)、臨床化学(4年)、アドバンスト薬物治療学1(4年)、病態・薬物治療学演習(4年)、特別演習・実習(4年~6年)、薬局方総論(6年)、薬学総合演習(6年)
病態評価演習(大学院)
■所属学会および社会活動
日本薬学会、日本分析化学会 -
柳田 寛太
講師 / 博士(理学)
■担当科目
医療薬学導入学習(1年)、薬物治療学3(3年)、病態・薬物治療学演習(4年)、特別演習・実習(4~6年)
病態評価演習(大学院生)
■所属学会および社会活動
日本認知症学会、日本薬学会
研究内容(薬物治療学II研究室)
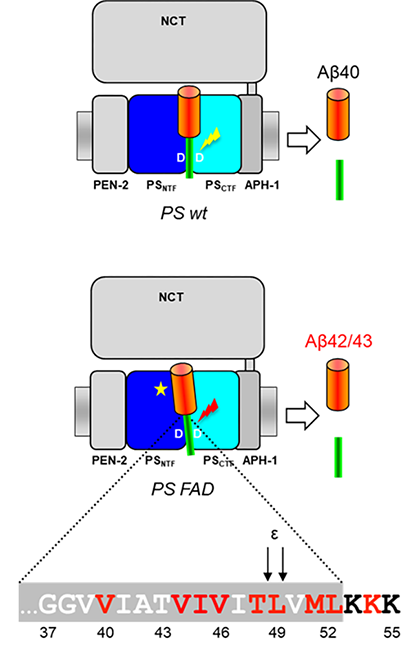
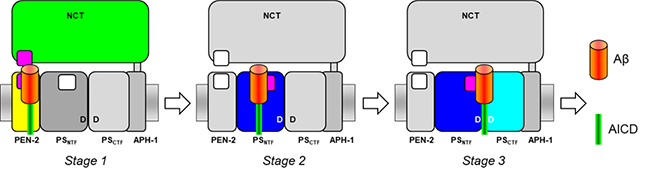
(1)アルツハイマー病の分子病態解明及びその応用に関する研究
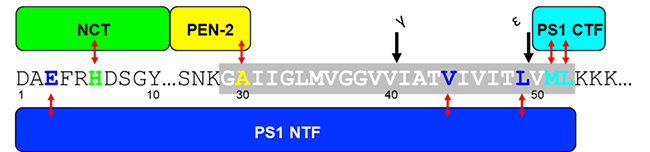
②家族性アルツハイマー病のプレセニリン変異体での酵素と基質のアミノ酸レベルの相互作用の変化
③基質のエクソサイトから触媒部位へのダイナミックな輸送
参考文献
- Fukumori, A., Feilen, L. P., and Steiner, H. (2020) Substrate recruitment by γ-secretase. Semin. Cell Dev. Biol. 10.1016/j.semcdb.2020.03.006
- Trambauer, J., Fukumori, A., and Steiner, H. (2020) Pathogenic Aβ generation in familial Alzheimer’s disease: novel mechanistic insights and therapeutic implications. Curr. Opin. Neurobiol. 61, 73–81
- Steiner, H., Fukumori, A., Tagami, S., and Okochi, M. (2018) Making the final cut : pathogenic amyloid- β peptide generation by γ -secretase. Cell Stress. 2, 292–310
- Trambauer, J., Fukumori, A., Kretner, B., and Steiner, H. (2017) Analyzing Amyloid-β Peptide Modulation Profiles and Binding Sites of γ-Secretase Modulators. Methods Enzymol. 584, 157–183
- Fukumori, A., and Steiner, H. (2016) Substrate recruitment of γ‐secretase and mechanism of clinical presenilin mutations revealed by photoaffinity mapping. EMBO J. 35, 1628–1643
(2)非天然アミノ酸の遺伝的取り込み法を用いたアミノ酸レベルでの相互作用の解析とその応用
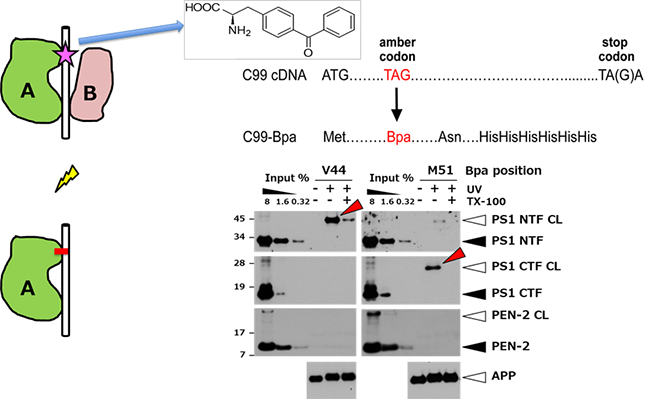
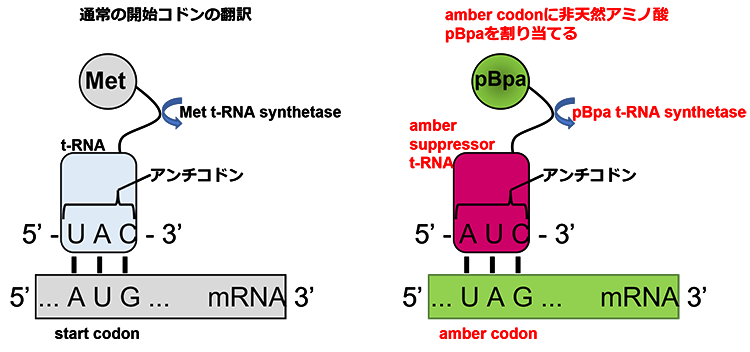
参考文献
- Fukumori, A., and Steiner, H. (2016) Substrate recruitment of γ‐secretase and mechanism of clinical presenilin mutations revealed by photoaffinity mapping. EMBO J. 35, 1628–1643
- Chávez‐Gutiérrez, L., and De Strooper, B. (2016) Probing γ‐secretase–substrate interactions at the single amino acid residue level. EMBO J. 35, 1597–1599
- Chin, J. W., Martin, A. B., King, D. S., Wang, L., and Schultz, P. G. (2002) Addition of a photocrosslinking amino acid to the genetic code of Escherichiacoli. Proc. Natl. Acad. Sci. U. S. A. 99, 11020–4
- Fukumori, A., Trambauer, J., Feilen, L. P., and Steiner, H. (2018) Identifizierung von Substratbindestellen in der γ-Sekretase. BioSpektrum. 24, 34–36 次のホームページで閲覧可能 http://rdcu.be/GLyg
(3) 病態関連分子及びその相互作用の化学的可視化に関する研究
医薬品など)の新規高感度分析法の開発について キサンテン系色素誘導体(下図参照)と金属との三元錯体生成反応などをもとに検討する。
作用に関する探索について検討する。
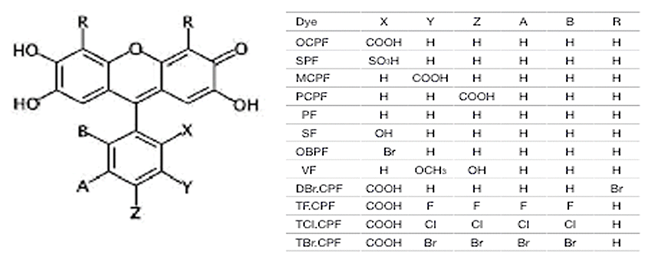
Structure of o-Carboxyphenylfluorone as a Multifunctional Dye. X-ray Structure Analysis online, 32, 9-10
次のホームページで閲覧可能 https://www.jstage.jst.go.jp/article/xraystruct/32/0/32_9/_article/-char/ja/
2.Ikeda C., Nakahara R., Nishioka Y., Kurokawa H., Yamaguchi T., Fujita Y. (2009) Spectrophotometric Determination of
Nitrite Ion with Pyrogallol Red-Molybdenum(VI) Complex. BUNSEKI KAGAKU, 8, 675–379
(4) その他、
代表的論文
2025年
- Stephan Breimann., Frits Kamp., Gabriele Basset., Claudia Abou-Ajram., Gökhan Güner., Kanta Yanagida., Masayasu Okochi., Stephan A Müller., Stefan F Lichtenthaler., Dieter Langosch., Dmitrij Frishman., Harald Steiner. (2025) Charting γ-secretase substrates by explainable AI. Nat Commun. 16(1):5428.
- Maruyama R., Fukumori A., Funamoto S., Okada K., Akamine S., Yanagida K., Shinohara M., Sato N., Okochi M., Kudo T., Steiner H. (2025) γ-Secretase exosites as targets for substrate-selective lowering of Aβ generation. Structure. S0969-2126(25)00444-7.
2023年
- Yanagida K., Maruyama R., Tagami S., Kudo T., Okochi M., Fukumori A.(2023)APLP2 is predominantly cleaved by β-secretase and γ-secretase in the human brain. Psychogeriatrics. 23(2):311-318.
- Matsunaga H., Fukumori A., Mori K., Morihara T., Sato S., Kitauchi K., Yanagida K., Taguchi K., Honda T., Tomonaga K.(2023)Ribavirin Treatment for Severe Schizophrenia with Anti-Borna Disease Virus 1 Antibodies 30 Years after Onset. Case Rep Psychiatry. 2023:4899364.
2022年
- Feilen LP., Chen SY., Fukumori A., Feederle R., Zacharias M., Steiner H.(2022)Active site geometry stabilization of a presenilin homolog by the lipid bilayer promotes intramembrane proteolysis. Elife. 11:e76090.
2021年
- Shinohara, M., Hirokawa, J., Shimodaira, A., Tashiro, Y., Suzuki, K., Gheni, G., Fukumori, A., Matsubara, T., Morishima, M., Saito, Y., Murayama, S., Sato, N. (2021) ELISA Evaluation of Tau Accumulation in the Brains of Patients with Alzheimer Disease. J Neuropathol Exp Neurol. 80(7):652-662.
- Shinohara, M., Kikuchi, M., Onishi-Takeya, M., Tashiro, Y., Suzuki, K., Noda, Y., Takeda, S., Mukouzono, M., Nagano, S., Fukumori, A., Morishita, R., Nakaya, A., Sato, N. (2021) Upregulated expression of a subset of genes in APP;ob/ob mice: Evidence of an interaction between diabetes-linked obesity and Alzheimer's disease. FASEB Bioadv. 3(5):323-333.
- Suzuki, K., Shinohara, M., Uno, Y., Tashiro, Y., Gheni, G., Yamamoto, M., Fukumori, A., Shindo, A., Mashimo, T., Tomimoto, H., Sato, N. (2021) Deletion of B-cell translocation gene 2 (BTG2) alters the responses of glial cells in white matter to chronic cerebral hypoperfusion. J Neuroinflammation. 18(1):86.
- Adachi, H., Yamamoto, R., Fujino, R., Kanayama, D., Sakagami, Y., Akamine, S., Marutani, N., Yanagida, K., Mamiya, Y., Koyama, M., Shigedo, Y., Sugita, Y., Mashita, M., Nakano, N., Watanabe, K., Ikeda, M., Kudo, T. (2021) Association of weekday-to-weekend sleep differences and stress response among a Japanese working population: a cross-sectional study. Sleep Med. 82:159-164.
2020年
- Fukumori, A., Feilen, L. P., and Steiner, H. (2020) Substrate recruitment by γ-secretase. Semin. Cell Dev. Biol. 105:54-63
- Trambauer, J., Fukumori, A., and Steiner, H. (2020) Pathogenic Aβ generation in familial Alzheimer’s disease: novel mechanistic insights and therapeutic implications. Curr. Opin. Neurobiol. 61: 73–81
- Shinohara M, Tashiro Y, Suzuki K, Fukumori A, Bu G, Sato N. (2020) Interaction between APOE genotype and diabetes in cognitive decline. Alzheimers Dement (Amst). 12(1):e12006.
- Shinohara M, Tashiro Y, Shinohara M, Hirokawa J, Suzuki K, Onishi-Takeya M, Mukouzono M, Takeda S, Saito T, Fukumori A, Saido TC, Morishita R, Sato N. (2020) Increased levels of Aβ42 decrease the lifespan of ob/ob mice with dysregulation of microglia and astrocytes. FASEB J. 34(2):2425-2435.
- Trambauer J, Rodríguez Sarmiento RM, Fukumori A, Feederle R, Baumann K, Steiner H. (2020) Aβ43-producing PS1 FAD mutants cause altered substrate interactions and respond to γ-secretase modulation. EMBO Rep. 21(1):e47996.
- Akamine S, Marutani N, Kanayama D, Gotoh S, Maruyama R, Yanagida K, Sakagami Y, Mori K, Adachi H, Kozawa J, Maeda N, Otsuki M, Matsuoka T, Iwahashi H, Shimomura I, Ikeda M, Kudo T. (2020) Renal function is associated with blood neurofilament light chain level in older adults. Sci Rep. 10(1):20350.
- Matsuhisa K, Saito A, Cai L, Kaneko M, Okamoto T, Sakaue F, Asada R, Urano F, Yanagida K, Okochi M, Kudo Y, Matsumoto M, Nakayama KI, Imaizumi K. (2020) Production of BBF2H7-derived small peptide fragments via endoplasmic reticulum stress-dependent regulated intramembrane proteolysis. FASEB J. 34(1):865-880.
2019年
- Mentrup, T., Theodorou, K., Cabrera-Cabrera, F., Helbig, A. O., Happ, K., Gijbels, M., Gradtke, A.-C., Rabe, B., Fukumori, A., Steiner, H., Tholey, A., Fluhrer, R., Donners, M., and Schröder, B. (2019) Atherogenic LOX-1 signaling is controlled by SPPL2-mediated intramembrane proteolysis. J. Exp. Med. 216 (4): 807–830.
2018年
- Fukumori, A., Trambauer, J., Feilen, L. P., and Steiner, H. (2018) Identifizierung von Substratbindestellen in der γ-Sekretase. BioSpektrum. 24, 34–36
- Matsunaga, H., Fukumori, A., Mori, K., Honda, T., Uema, T., and Tomonaga, K. (2018) Two neuropsychiatric cases seropositive for bornavirus improved by ribavirin. Jpn. J. Infect. Dis. 71, 338–342
- Steiner, H., Fukumori, A., Tagami, S., and Okochi, M. (2018) Making the final cut : pathogenic amyloid- β peptide generation by γ -secretase. Cell Stress. 2, 292–310